
Ethylene Glycol Diol C2h6o2 Molecule Used Stock Vector (Royalty Free) 1718457154 Shutterstock
Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in C2H6O2: Molar Mass (g/mol) C (Carbon) 2 × 12.0107 = 24.0214. H (Hydrogen) 6 × 1.00794 = 6.04764. O (Oxygen)

Balancing the Equation C2H6O2 + O2 = CO2 + H2O (and Type of Reaction) YouTube
Methoxymethanol | C2H6O2 | CID 62540 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety.

C2H6O2 C2H6O2 JapaneseClass.jp
Ethylene glycol (IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula (CH 2 OH) 2.It is mainly used for two purposes, as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odorless, colorless, flammable, viscous liquid.

C2h6o2 ethylene glycol molecule Royalty Free Vector Image
The molecular formula C2H6O2 (molar mass: 62.07 g/mol, exact mass: 62.03678 u) may refer to: Ethylene glycol (ethane-1,2-diol) Ethyl hydroperoxide. Methoxymethanol. Dimethyl peroxide. This set index page lists chemical structure articles associated with the same molecular formula. If an internal link led you here, you may wish to change the.

Dihydroxy Alcohol C2H6O2 Meg Mono Ethylene Glycol For Antifreeze Formulations
1,1-Ethanediol | C2H6O2 | CID 151211 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety.
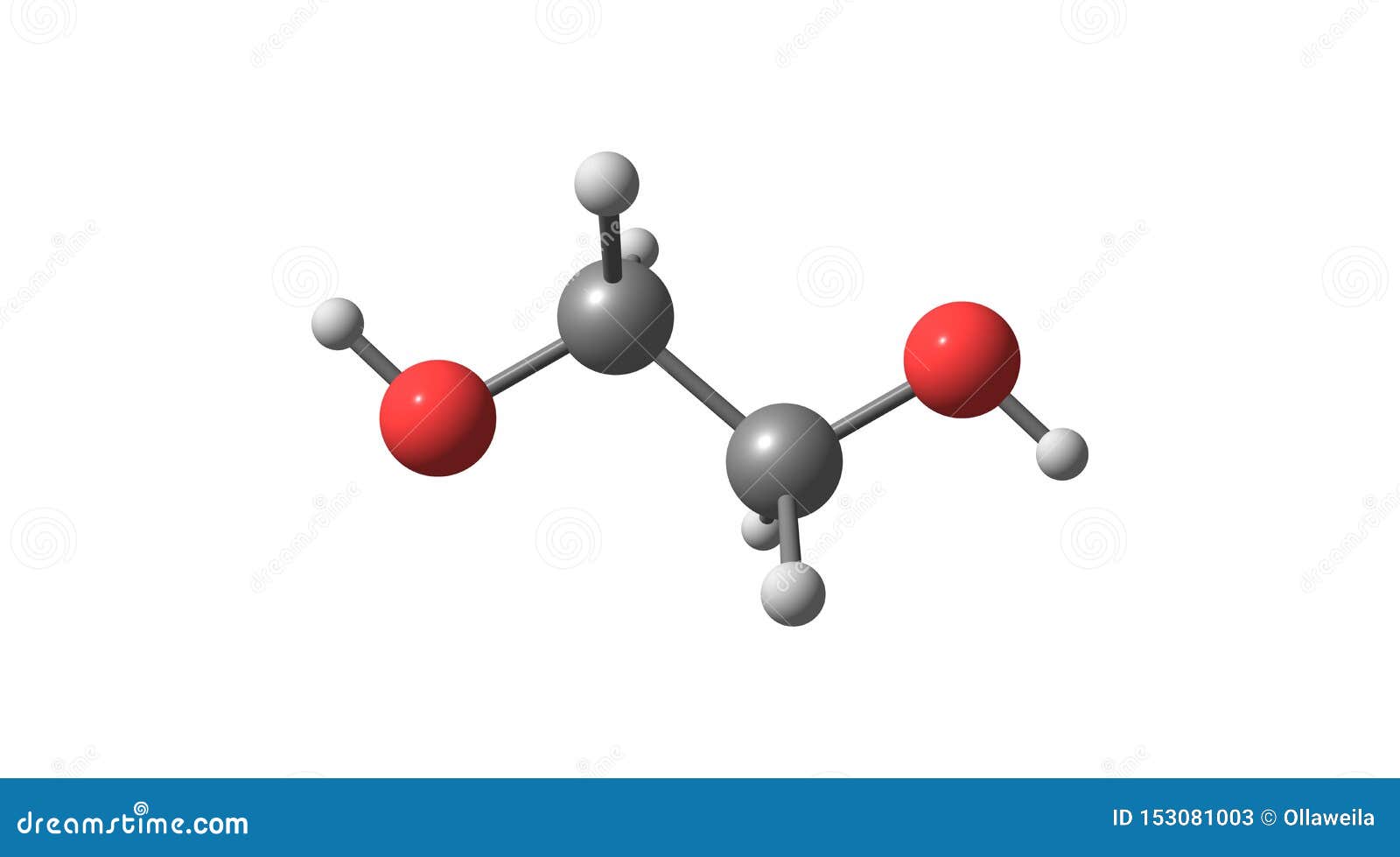
Ethylene Glycol, Diol, C2H6O2 Molecule. It Is Used For Manufacture Of Polyester Fibers And For
C2H6O2 molecular weight. Molar mass of C2H6O2 = 62.06784 g/mol. Convert grams C2H6O2 to moles. or. moles C2H6O2 to grams. Molecular weight calculation: 12.0107*2 + 1.00794*6 + 15.9994*2. Percent composition by element. Element: Hydrogen Symbol: H Atomic Mass: 1.00794 # of Atoms: 6 Mass Percent: 9.744%. Element: Carbon
Ethylene Glycol detection (C2H6O2) Gas Factsheet Ion Science UK
Explanation of how to find the molar mass of C2H6O2 or (CH₂OH)2 : Ethylene glycol.A few things to consider when finding the molar mass for C2H6O2 or (CH₂OH)2.
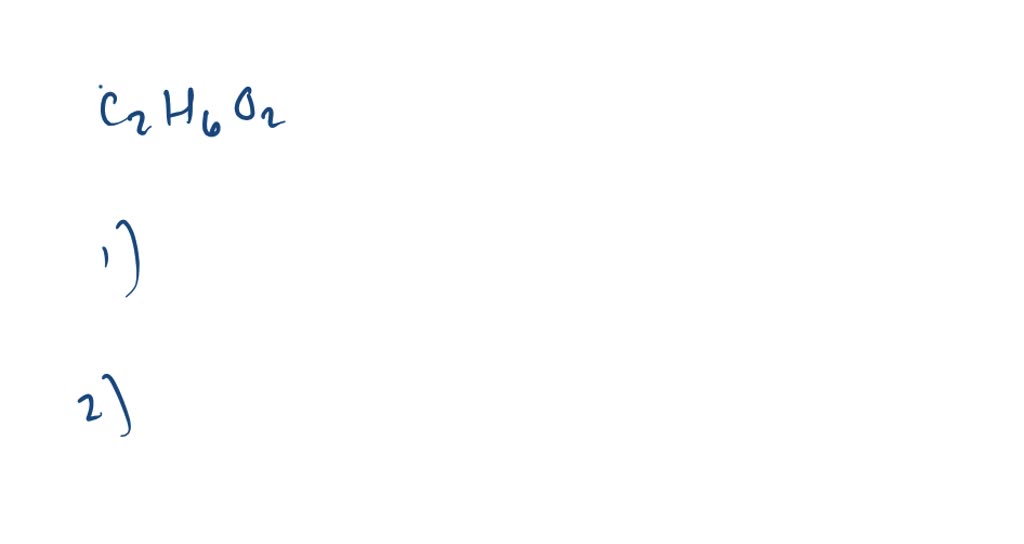
SOLVED Write the condensed structures of all isomers with the formula C2H6O2. Label the
Molar Mass, Molecular Weight and Elemental Composition Calculator. Molar mass of (mr)C2H6O is 46.0684 g/mol. Get control of 2022! Track your food intake, exercise, sleep and meditation for free. Convert between (mr)C2H6O weight and moles. Compound. Moles.

Friction coefficient of C2H6O2 with three different concentrations... Download Scientific Diagram
Learning Objectives. To describe the relationship between solute concentration and the physical properties of a solution. To understand that the total number of nonvolatile solute particles determines the decrease in vapor pressure, increase in boiling point, and decrease in freezing point of a solution versus the pure solvent.

Draw Possible Isomers Of C2H6O2 YouTube
Methyl acrylate, stabilized is a colorless volatile liquid with an acrid odor. Flash point 27 °F. Vapors may irritate the eyes and respiratory system. Highly toxic by inhalation, ingestion and skin absorption. Less dense than water (0.957 gm / cm3) and slightly soluble in water, hence floats on water.
[Solved] Write the Lewis structure for ethylene glycol, C2H6O2, the main... Course Hero
Mr C₂H₆O₂ = 2.Ar C + 6.Ar H + 2.Ar O. Mr C₂H₆O₂ = 2 (12) + 6 (1) + 2 (16) Mr C₂H₆O₂ = 24 + 6 + 32. Jadi massa molekul relatif etilen glikol C₂H₆O₂ adalah Mr = 62. 5 votes Thanks 5. More Questions From This User See All. iim04 September 2018 | 0 Replies.

C2H6O2 C2H6O2 JapaneseClass.jp
15.9994. 3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in C2H6O: Molar Mass (g/mol) C (Carbon) 2 × 12.0107 = 24.0214. H (Hydrogen) 6 × 1.00794 = 6.04764.
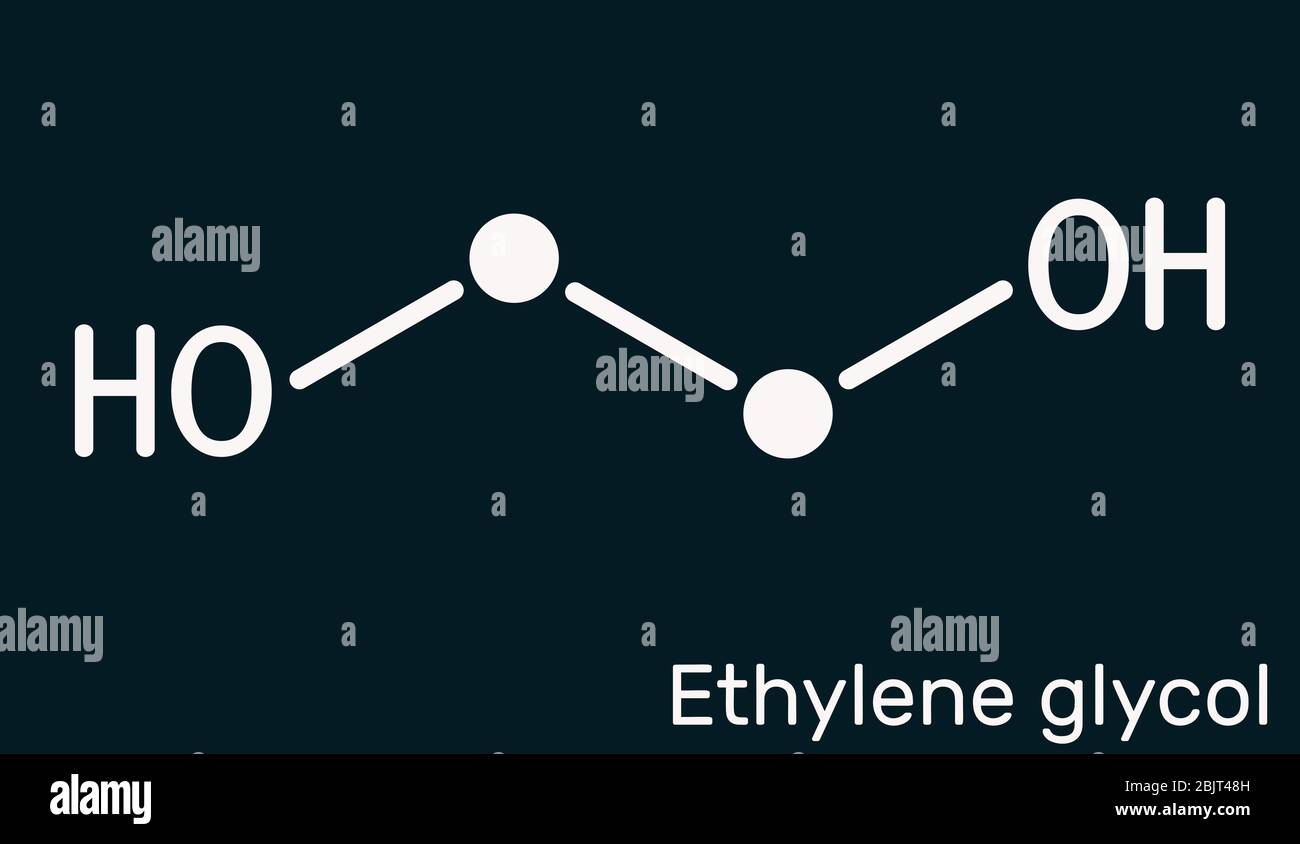
Ethylene glycol, diol, C2H6O2 molecule. It is used for manufacture of polyester fibers and for
methyl peroxide. Molecular Formula CHO. Average mass 62.068 Da. Monoisotopic mass 62.036777 Da. ChemSpider ID 120261.

Calculate the mole fraction of ethylene glycol in a solution containing 20 of C2H6O2 by mass
Found 8 results. Search term: C2H6O2 (Found by molecular formula) ID. Structure. Molecular Formula. Molecular Weight. # of Data Sources. # of References. # of PubMed.
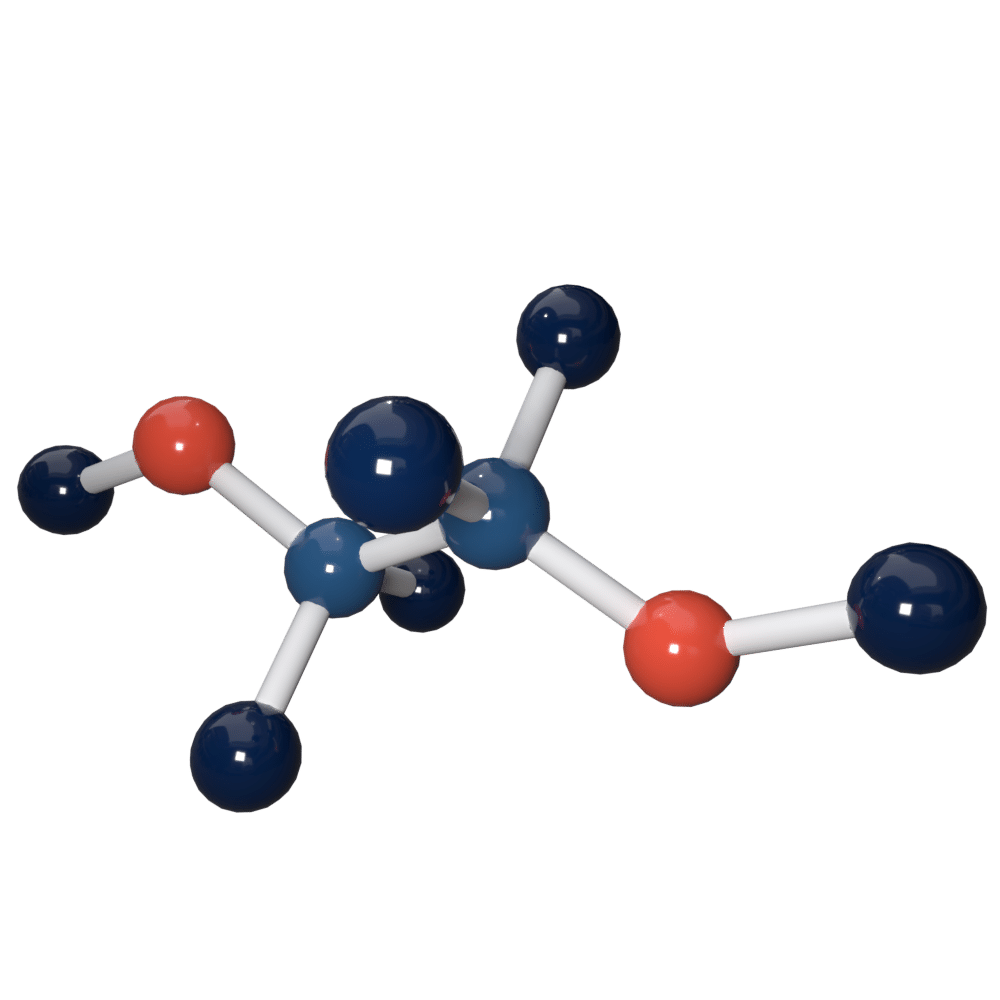
Detecting Ethylene Glycol (C2H6O2) Gas Factsheet Ion Science UK
Ethylene glycol is a synthetic liquid substance that absorbs water. It is odorless, but has a sweet taste. Ethylene glycol is used to make antifreeze and de-icing solutions for cars, airplanes, and boats. It is also used in hydraulic brake fluids and inks used in stamp pads, ballpoint pens, and print shops.

Calculate the mole fraction of ethylene glycol (C2H6O2) in a solution containing 20
So, Molar mass of Ethylene glycol (C2H6O2) = Molar mass of 2 Carbon (C) atoms + Molar mass of 6 Hydrogen (H) atoms + Molar mass of 2 Oxygen (O) atoms. Hence the Molar mass of Ethylene glycol (C2H6O2) is 62.068 g/mol. I hope you have understood the short and simple calculation for finding the molar mass of Ethylene glycol.