
A, B and C are three biomolecules. the results of teh tests performed on them are given below
Molisch's test is a qualitative test used to detect the presence of aldehydes and ketones in a sample. The test uses a reagent made up of Schiff's reagent and concentrated sulfuric acid. When the reagent is added to a sample containing aldehydes or ketones, the aldehydes or ketones will react with the Schiff's reagent to form a colored.
PPT Lab Activity1 Carbohydrates PowerPoint Presentation, free download ID1998637
Identification Tests for Carbohydrates (Playlist 👇🏻)https://www.youtube.com/watch?v=TB7lbHTOoh0&list=PLEIbY8S8u_DJunHAPAJ8_GcQQ1Rbn1NMVBasics of Analytical.

01 Réaction de MOLISCH YouTube
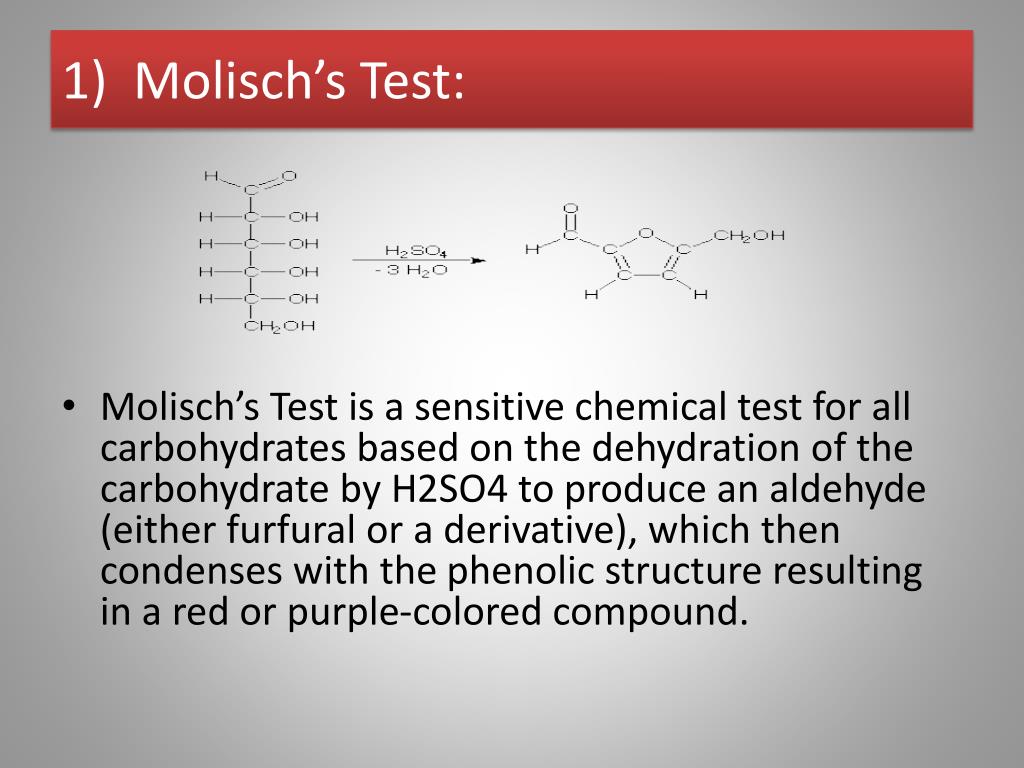
Molisch's test is a general test for all carbohydrates. In this test, carbohydrates when reacted with conc. H2SO4 get dehydrated to form furfural and its derivatives. When monosaccharide are treated with conc H2SO4 or conc HCl, -OH group of sugar are removed in the form of water and furfural is formed from pentose sugar and hydroxymethyl furfural is formed from hexose sugar.

Molisch’s Test Objectives, Principle, Reagents, Procedure and Result Online Biology Notes
Quick Reference. A biochemical test to detect the presence of carbohydrates in solution, also known as Molisch's test (after the Austrian chemist H. Molisch (1856-1937), who devised it). A small amount of alcoholic alpha-naphthol is mixed with the test solution and concentrated sulphuric acid is poured slowly down the side of the test tube.

Molisch's Test Practical Experiment YouTube
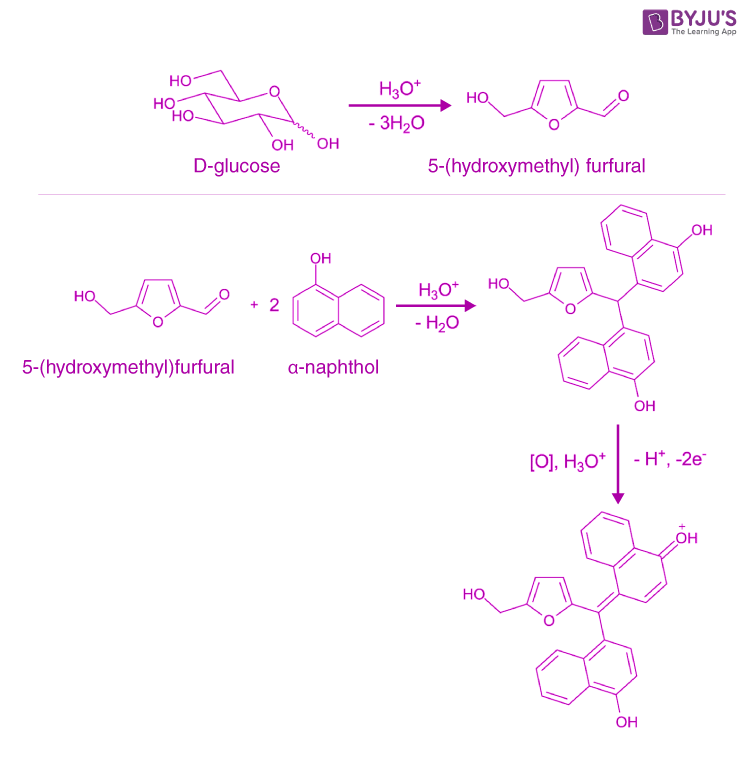
Molisch Test Principle. During the reaction, applying sulphuric acid can remove the water molecules from the given carbohydrate; hence aldehyde is generated. The α-naphthol in molischs reagent undergoes condensation with the aldehyde, forming a reddish-purple-coloured complex. A detailed feature of the Molisch Test on D-glucose is provided.

Practical Biochemistry
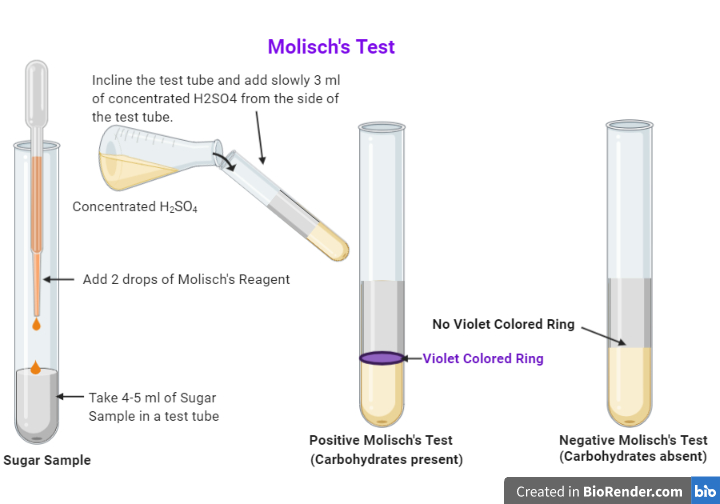
Result of Molisch's Test. A purple/violet colored ring is observed at the junction of the two liquids, i.e., Molisch's test reagent and dehydrated carbohydrate solution. In there is no ring, then the given sample does not contain any carbohydrates [1]. A positive reaction for Molisch's test is given by almost all carbohydrates, except.

Reacción de Molish Mecanismo Completo Identificación de carbohidratos YouTube
Molisch's Test Procedure. 2-3 drops of Molisch's reagent must be added to a small amount of the analyte in a test tube and mixed well. Now, a few drops of concentrated sulphuric acid must be added drop-wise along the walls of the test tube to facilitate the formation of a layer and avoid mixing. The development of a purple ring at the layer.

Molisch's Test Definition, Reaction, Reagent, and Procedure
Therefore, Molisch's test is very important for the detection of the presence of carbohydrates in a substance. Principle of Molisch Test. The reaction is generally based on the fact that the concentrated acid helps in catalysing the dehydration of sugars to form furfural (via pentoses) or hydroxymethylfurfural (via hexoses).
Molisch's Test Definition, Principle, Procedure, Result, Uses
: After the discovery in the year 1886, Molisch's reaction used to be applied as a powerful tool to differentiate carbohydrates and glycosides qualitatively from other materials. A brief review is given herein on the discovery, reaction mechanism and application of Molisch's reaction. Perspectives are also given on the potential applications of Molisch's reaction.

Molisch's test Wikipedia
What is Molisch's Test? Molisch test is a colourimetric method for the analysis of the presence of carbohydrates in a given analyte. This test is named after Austrian botanist Hans Molisch. Molisch's test is done by using Molisch reagent. A solution of naphthol in ethanol (95%) is known as Molisch reagent. It's also known as the purple.

Figure 1. Molisch test for Carbohydrates Laboratory Activities to Introduce Carbohydrates
Reactions: The test reagent dehydrates pentoses to form furfural (top reaction) and dehydrates hexoses to form 5-hydroxymethyl furfural (bottom reaction).. Two drops of the Molisch reagent (a solution of -napthol in 95% ethanol) is added. The solution is then poured slowly into a tube containing two ml of concentrated sulfuric acid so that.
Molisch’s Test Principle, Procedure, Reaction, & Reagent Preparation
Molisch's reaction. Molisch's test: reactives, sulphuric acid and 2% -naphtol in ethanol (a) and steps of procedure of application (b and c).

PPT Lecture 1. WET METHODS OF CARBOHYDRATE ANALYSES PowerPoint Presentation ID323603
The Molisch test is a highly sensitive colorimetric method used to detect the presence of carbohydrates, whether they are free or bound to proteins or lipids. It involves using a solution of α-naphthol (C 10 H 8 OH) in 95% ethanol (C 2 H 5 OH), which is known as the Molisch reagent . The formation of a purple or purplish-red ring at the site.

molisch反应呈现反应的成分有哪些(molisch反应过程)
Request PDF | On Jan 1, 2021, Zhiyou Hao and others published Molisch's Reaction: Discovery, Mechanism and Application | Find, read and cite all the research you need on ResearchGate

Molisch’s Test Objectives, Principle, Reagents, Procedure and Result
Molisch test is a group test for all carbohydrates, either free or bound to proteins or lipids.. so they do not give a positive result for this reaction. Molisch test is not a specific test for carbohydrates. Furfurals as such or furfural yielding substance, some organic acids like citric acids, lactic acid, oxalic acid, formic acid, etc.

PPT Lecture 1. WET METHODS OF CARBOHYDRATE ANALYSES PowerPoint Presentation ID323603
PROCEDURE: To 2ml of sugar solution (original solution) add 2 to 3 drops of Molisch's reagent. Mix thoroughly. Carefully pour 5 ml concentrated H2SO4 along the side of the test tube. Acid being heavier will form a layer beneath the sugar solution.