
Nitrogen Moregas
Nitrogen Quantity Conversions Calculator. Scf (standard cubic foot) gas measured at 1 atmosphere and 70°F. Nm3 (normal cubic meter) gas measured at 1 atmosphere and 0°C. Liquid measured at 1 atmosphere and boiling temperature. Nitrogen quantity units converter. Supplier of new and used industrial gas plants and plant components plus related.

Jual Tabung Gas Nitrogen Murah , Jual Gas Nitrogen Murah, Jual Tabung Nitrogen Murah, Jual
Jadi, massa dari 10 liter gas nitrogen (N2) pada 27°C, 2 atm adalah 22,764 gram. Pada suhu dan tekanan di luar keadaan standar atau keadaan kamar, volume gas dapat ditentukan dengan persamaan gas ideal. Rumus: PV = nRT. n n N2 m Mr N2 Massa N2 = = = = = = = = = = RTPV 0,082 L atm mol−1K−1x (273+27) K2 atm x 10 L 0,082 L atm mol−1K−1x.

99.9 5 Kg Nitrogen Gas, 183 Degree C at best price in Ahmedabad ID 22433105655
As your nitrogen gas supplier of choice, our team can meet your exact application needs. Connect with us soon and ask about next-day nitrogen gas delivery in Los Angeles or Orange County. Get A Quote.. VGL 160 Liters. Silver. NI FOD160LT22. 20"W x 64"H. VGL 160 Liters. Silver. NI NF160LT22.

Jual Tabung Gas Nitrogen 1m3 di Lapak Sentra Gas Industri Bukalapak
The second method is to us Avogadro's Volume at STP 22.4L = 1mol. In both cases the significant digit count is three figures which means the acceptable solution is 125L. I hope this was helpful. There are two ways to calculate this if we assume STP of 1 atm and 273 K for the pressure and temperature. We can use the Ideal Gas Law equations PV.

How and Why Is Nitrogen Gas Used For Packaging?
About Nitrogen gas; Nitrogen gas weighs 0.001251 gram per cubic centimeter or 1.251 kilogram per cubic meter, i.e. density of nitrogen gas is equal to 1.251 kg/m³; at 0°C (32°F or 273.15K) at standard atmospheric pressure.In Imperial or US customary measurement system, the density is equal to 0.0781 pound per cubic foot [lb/ft³], or 0.00072312 ounce per cubic inch [oz/inch³] .

🔴[STOIKIOMETRI]🔴Pada suhu dan tekanan tertentu massa dari 5 liter gas nitrogen adalah 7 gram
Calculate pressure, volume, quantity (moles) or temperature of a gas with this versatile Ideal Gas Laws calculator (moles) by entering the other three. Free online gas law calculator a.k.a. PV = nRT calculator which accepts different input metric units such as temperature in celsius, fahrenheit, kelvin; pressure in pascals, bars, atmospheres; volume in both metric and imperial units cubed.

Nitrogen Gas, For In Laboratory at Rs 400/cubic meter in Bengaluru ID 15678722333
To find any of these values, simply enter the other ones into the ideal gas law calculator. For example, if you want to calculate the volume of 40 moles of a gas under a pressure of 1013 hPa and at a temperature of 250 K, the result will be equal to: V = nRT/p = 40 × 8.31446261815324 × 250 / 101300 = 0.82 m³.

Pembakaran gas nitrogen terjadi menurut reaksi berikut.N2...
This online calculator converts grams to liters and liters to grams given a gas formula. It uses molar volume of a gas at STP (standard temperature and pressure) This calculator finishes the topic started in Convert moles to liters and liters to moles calculator. Because the molar volume is the same for all ideal gases and is known, we can.

Jual Gas Nitrogen, Jual Gas Nitrogen di Bandung, Jual Gas Nitrogen di Medan, Jual Nitrogen Murah
Share. We offer compressed nitrogen gas and liquid nitrogen (N 2) in a variety of purities and concentrations. Download Safety Data Sheets or see the chart below to download the spec sheets for more information on buying liquid nitrogen and nitrogen gas. Product Name. Concentration. Spec Sheet. Compressed Nitrogen Gas (N 2) High Purity > 99.998%.
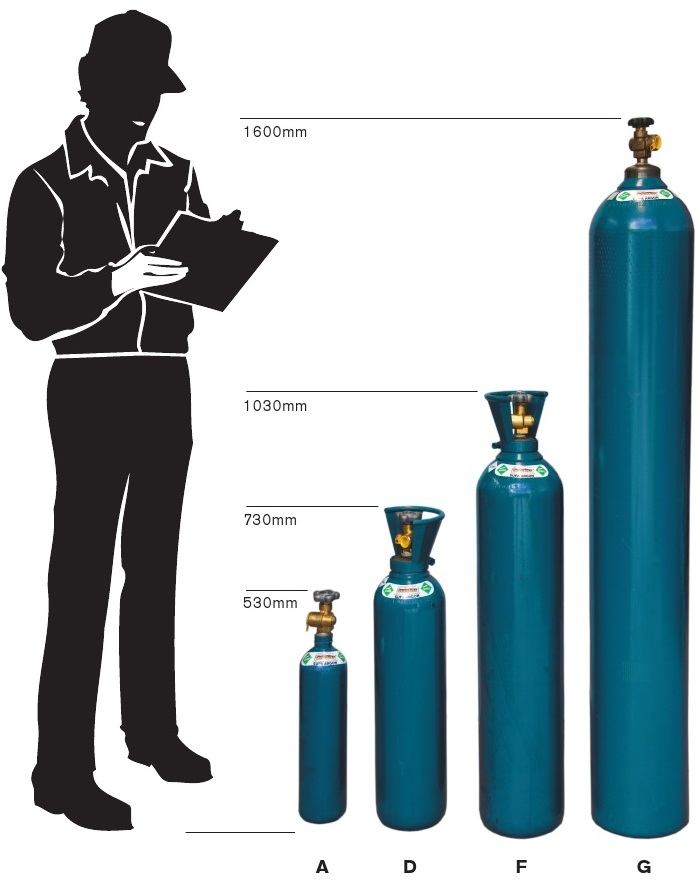
Nitrogen Gas Boc Nitrogen Gas Bottle Sizes
The mass of 0.560 moles of chlorine gas Cl 2 is 39.71 g. To obtain this value, follow these steps: Determine the molar mass of the gas. Since chlorine has a molar mass of 35.453 g/mol on the periodic table, the molar mass of the chlorine gas Cl 2 is twice this value. This is 70.906 g/mol. Use the molar mass formula to calculate the mass:

Nitrogen Gas Phnom Penh Gas Center
To determine this value, we rearrange the ideal gas equation to. n V = P RT (10.5.1) (10.5.1) n V = P R T. Density of a gas is generally expressed in g/L (mass over volume). Multiplication of the left and right sides of Equation 10.5.1 10.5.1 by the molar mass in g/mol ( M M) of the gas gives.

99.9 Nitrogen Gas Cylinder, Rs 35 /cubic meter HP Industrial gases ID 23007247488
0.2. US teaspoon. 0.2. About Nitrogen gas. 1 cubic meter of Nitrogen gas weighs 1.251 kilograms [kg] 1 cubic inch of Nitrogen gas weighs 0.000723124 ounce [oz] Nitrogen gas weighs 0.001251 gram per cubic centimeter or 1.251 kilogram per cubic meter, i.e. density of nitrogen gas is equal to 1.251 kg/m³; at 0°C (32°F or 273.15K) at standard.

Nitrogen Gases, नाइट्रोजेन गैस in Noida , Nova Industrial Gases ID 8551831133
Nitrogen Weight and Volume Equivalents. Weight of Liquid or Gas. Volume of Liquid at Normal Boiling Point. Volume of Gas at 70°F (21°C) and 1 atm. lb.

Jual Gas Nitrogen UHP, N2, Jual Gas Nitrogen Food Grade, Jual N2, Jual Nitrogen N2, Harga
VIDEO ANSWER: So in this problem we want to find a mass of nitrogen dioxide gas given these conditions. This is uh and basic employment of the ideal gas law. And it is probably easier if you rearrange the constants to get in which is the mold of. Berapa massa dari 5 liter gas nitrogen ( N2, Mr=28) pada 27° C dan tekanan 3 ATM? Instant Video.

Spesifikasi Tabung Gas Nitrogen
About Nitrogen gas; Nitrogen gas weighs 0.001251 gram per cubic centimeter or 1.251 kilogram per cubic meter, i.e. density of nitrogen gas is equal to 1.251 kg/m³; at 0°C (32°F or 273.15K) at standard atmospheric pressure.In Imperial or US customary measurement system, the density is equal to 0.0781 pound per cubic foot [lb/ft³], or 0.00072312 ounce per cubic inch [oz/inch³] .

Nitrogen Gas Cylinder Food Grade at Rs 900/piece Nitrogen Gas Cylinder in Gurgaon ID
Beranda. Massa 5 liter nitrogen pada T dan P tertentu adala. Iklan. Pertanyaan. Massa 5 liter nitrogen pada T dan P tertentu adalah 5,6 gram. Berapa jumlah atom He yang terdapat dalam 10 liter gas He pada T dan P tersebut? (tetapan Avogadro = 6×1023; Ar N = 14) 1,2× 1023 atom. 2,4× 1023 atom. 2,7× 1023 atom.