
Perhatikan tabel berikut! No Garam Hasil Reaksi Hidrolisi...
El carbonato de sodio (Na2CO3) es una sal inorgánica de sodio, de metal alcalino y del ácido carbónico. También se le conoce mundialmente como ceniza de sosa. Los lagos y las actividades volcánicas enriquecieron de sodio los suelos, de los cuales se nutrieron las plantas; luego, tras un incendio, estas plantas esparcían las cenizas de carbonato.

Tipos de Hidrolisis
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. 2 HCl + Na2CO3 = 2 NaCl + H2O + CO2. Reactants.

Type of Reaction for NaHCO3 = Na2CO3 + H2O + CO2 YouTube
NaHCO3 = Na2CO3 + H2O + CO2 is a Decomposition reaction where two moles of Sodium Bicarbonate [NaHCO 3] decomposes into one mole of Sodium Carbonate [Na 2 CO 3], one mole of Water [H 2 O] and one mole of Carbon Dioxide [CO 2] Show Chemical Structure Image. Reaction Type. Decomposition.

PPT Types of Chemical Reactions PowerPoint Presentation, free download ID3726756
Hidrolisis - Kimia Kelas 11 - Teori, Jenis Reaksi, dan Contoh Soal. by sereliciouz & Andjar Tyassih, S.Si. Agustus 28, 2019. Dalam artikel ini akan dibahas secara detail tentang teori dan reaksi hidrolisis, jenis-jenis reaksi hidrolisis, contoh soal dan pembahasan reaksi hidrolisis, dan aplikasi reaksi hidrolisis dalam kehidupan sehari-hari.

Standardization Of HCl With Na2CO3; Standardization Of NaOH With Standard HCl1/ AcidBase
Na2CO3 (s) —-> 2 Na+(aq) + CO3-2(aq) The carbonate ion is able to remove protons (H+) from water to form bicarbonate ions and hydroxide ions. Hence it is the conjugate base of the bicarbonate ion;

TEM image after mixing of a) 40mM MgCl2 and 40mM Na2CO3, b) 40mM BaCl2... Download Scientific
Explicación sobre cómo encontrar el pH de una solución con doble hidrólisis del ácido carbónico asociado a la sal de carbonato de sodio (Na2CO3)utilizando la.

Hidrólisis qué es, cómo se lleva a cabo y cuál es su importancia Renovables Verdes
Question: What are the net ionic equations for the hydrolysis of the the following:NaC2H3O2Na2CO3NH4CLZnCl2KAl (SO4)2KAl (SO4)2 for 5 & 6 there are supposed to be 2 different hydrolysis reactions occuringAlso determine if each is Ka or Kb. What are the net ionic equations for the hydrolysis of the the following: Here's the best way to solve it.

Гидролиз солей презентация онлайн
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. Na2CO3 + 2 HCl = 2 NaCl + H2O + CO2. Reactants.

Na2CO3 + HCl YouTube
To tell if Na2CO3 (Sodium carbonate) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that N is a metal and CO3 is a g.
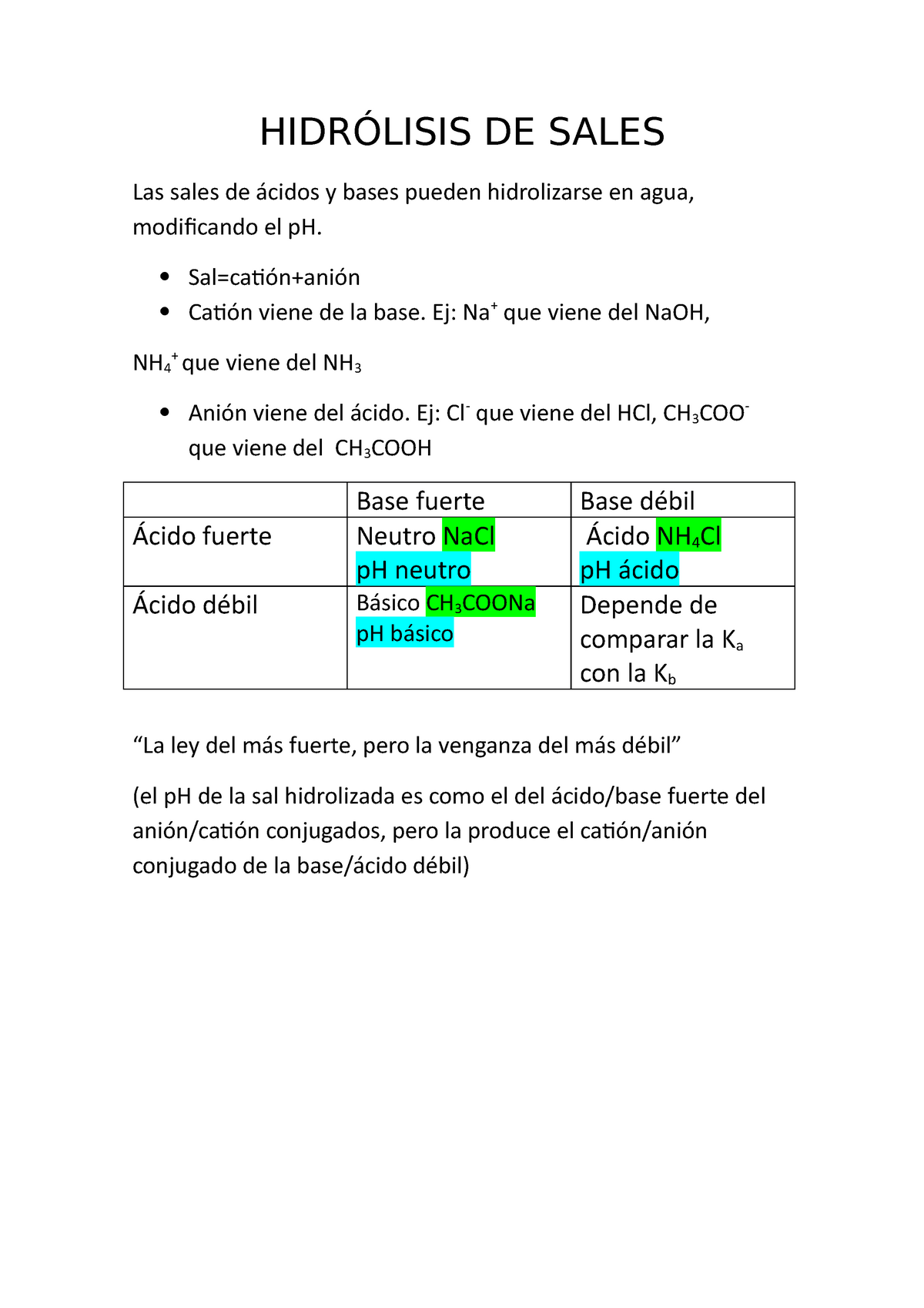
Hidrólisis DE Sales HIDRÓLISIS DE SALES Las sales de ácidos y bases pueden hidrolizarse en
In this video we will describe the equation Na2CO3 + H2O and write what happens when Na2CO3 is dissolved in water.When Na2CO3 is dissolved in H2O (water) it.

Hidrólisis acido base Hidrólisis Un ácido aporta protones, H+, al medio, una base aporta iones
Jawaban terverifikasi. Halo Siti A, kakak bantu jawab ya :) Reaksi hidrolisis pada Na2CO3 adalah CO32- + 2H2O ⇌ H2CO3 + 2OH-. Reaksi hidrolisis pada garam adalah reaksi pemecahan air oleh ion yang berasal dari asam lemah atau basa lemah yang terdapat pada garam. Na2CO3 → 2Na+ + CO32- Ion Na+ berasal dari basa kuat NaOH dan CO32- berasal.

Senyawa CaCl2, Na2CO3 , dan NaCl adalah garamgaram ya...
The ammonium ion is the conjugate acid of the base ammonia, NH 3; its acid ionization (or acid hydrolysis) reaction is represented by. NH4+(aq) + H2O(l) ⇌ H3O+(aq) + NH3(aq) Ka = Kw/Kb. Since ammonia is a weak base, Kb is measurable and Ka > 0 (ammonium ion is a weak acid). The chloride ion is the conjugate base of hydrochloric acid, and so.

TOMi.digital Síntesis y función biológica de los ácidos carboxílicos
El ion de amonio es el ácido conjugado de la base amoníaco, NH 3; su reacción de ionización ácida (o hidrólisis ácida) está representada por. NH4+(aq) + H2O(l) ⇌ H3O+(aq) + NH3(aq) Ka = Kw/Kb. Como el amoníaco es una base débil, Kb es medible y Ka > 0 (el ion de amonio es un ácido débil).
Práctica 6 Hidrólisis de una proteína y ensayos para proteínas y am…
Improve this question. This is the equation given by my textbook for hydrolysis of sodium carbonate: NaX2COX3 +2HX2O HX2COX3 +2NaX+ +2OHX− N a X 2 C O X 3 + 2 H X 2 O H X 2 C O X 3 + 2 N a X + + 2 O H X −. and it mentions that sodium ion (NaX+) ( N a X +) does not tend to combine with the hydroxide ion (OHX−) ( O H X −) and I was.
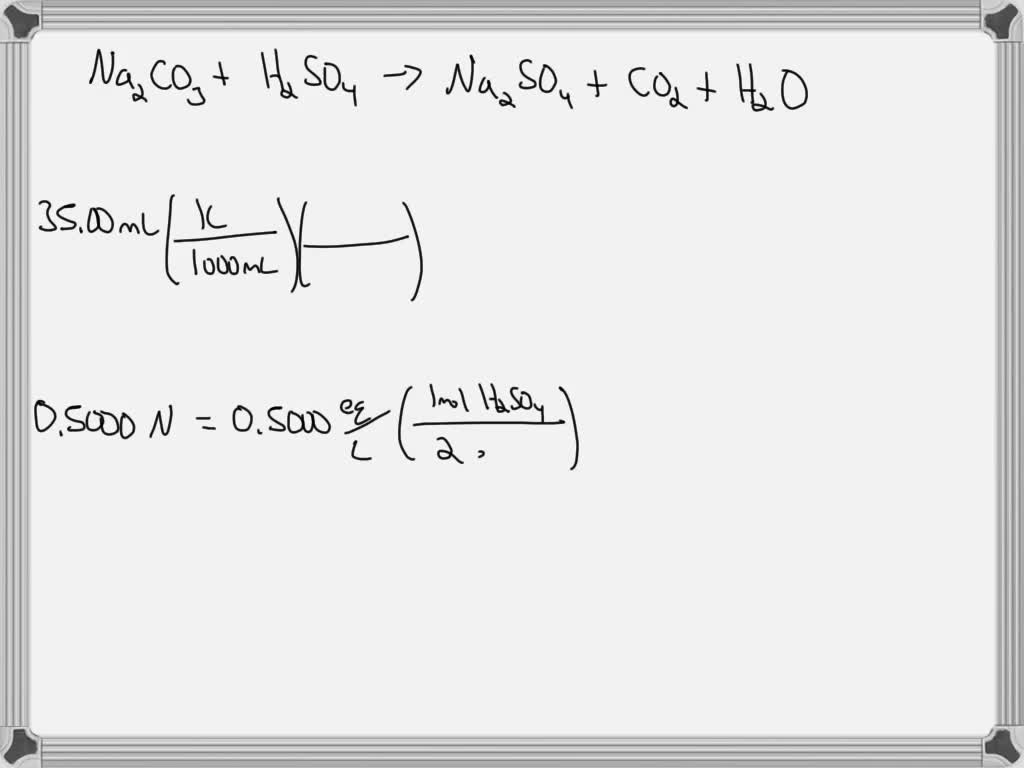
SOLVED Na2CO3 reacts with H2SO4 to give respective salt water and carbon dioxide. Calculate the
Sodium Carbonate Formula. Sodium carbonate is a diazonium salt of carbonic acid with the chemical formula Na2CO3. It is also known as Soda crystals, soda ash, washing soda. This inorganic compound is water-soluble and when dissolved in water, it forms carbonic acid and sodium hydroxide. In its pure form, it is a white powder and odourless.
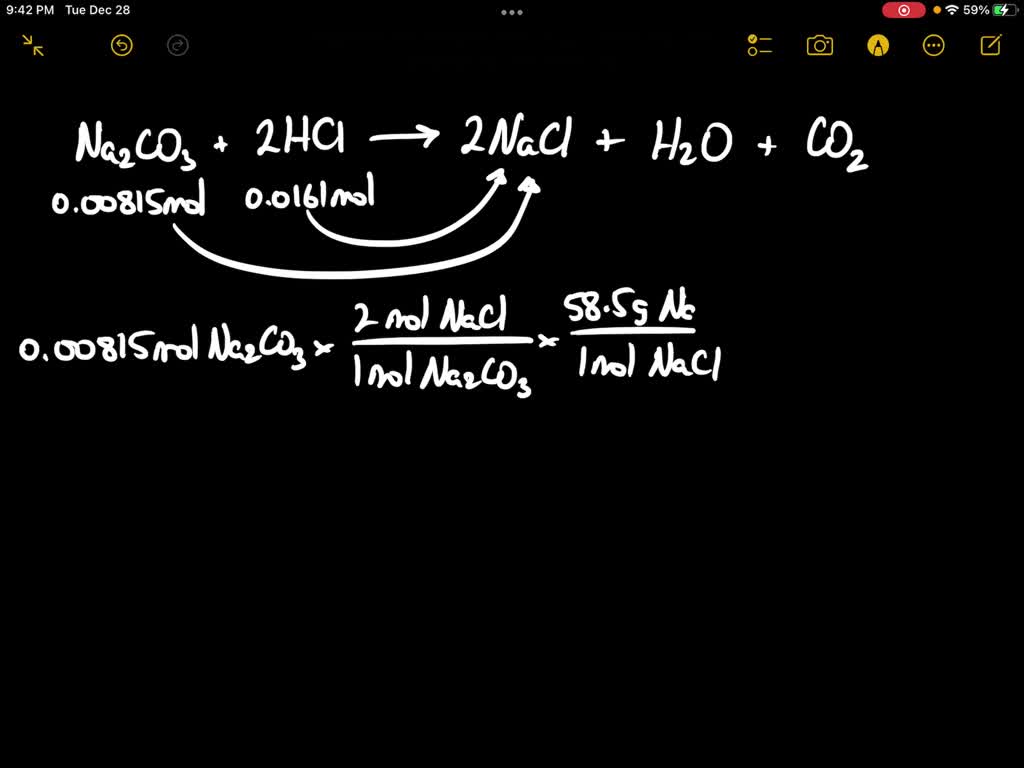
SOLVED A student reacts 0.00718 moles of sodium carbonate with 0.0136 moles of hydrochloric
Hydrolysis involving ionic compounds may be illustrated by the chemical changes occurring in an aqueous solution of the salt sodium acetate. In solution, the ionic constituents of the salt (the acetate ion and the sodium ion) separate; water molecules combine with the acetate ions to form acetic acid and hydroxide ions. Acetic acid dissociates reversibly into acetate ions and hydrogen ions.