
33+ de broglie wavelength calculator KeirraLeandro
Tako je De Brolj svojom hipotezom ukazao na teorijsku osnovu drugog Borovog postulata, da su orbite elektrona kvantizovane, odnosno da stabilna energetska stanja postoje samo ako je ispunjen uslov da je moment impulsa elektrona (L) jednak celobrojnom umnošku redukovane Plankove konstante ћ (čita se "ash").
.jpg)
Victor de Broglie Biography Topics referred to by the same term
Jaka jest hipoteza De Broglie? Hipoteza De Broglie sugeruje, że cała materia wykazuje właściwości falowe i wiąże obserwowaną długość fali materii z jej pędem. Menu Dom Nauka, technika, matematyka Nauki ścisłe Matematyka Nauki społeczne Informatyka Zwierzęta i przyroda Humanistyka Historia i kultura Dzieła wizualne Literatura język angielski

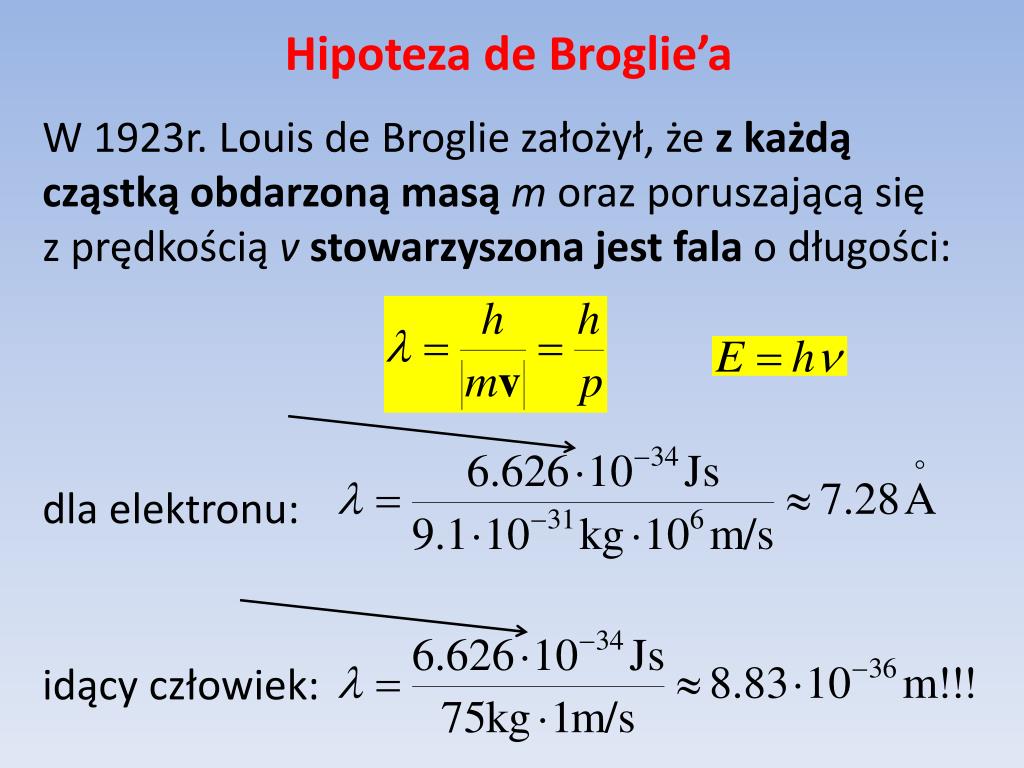
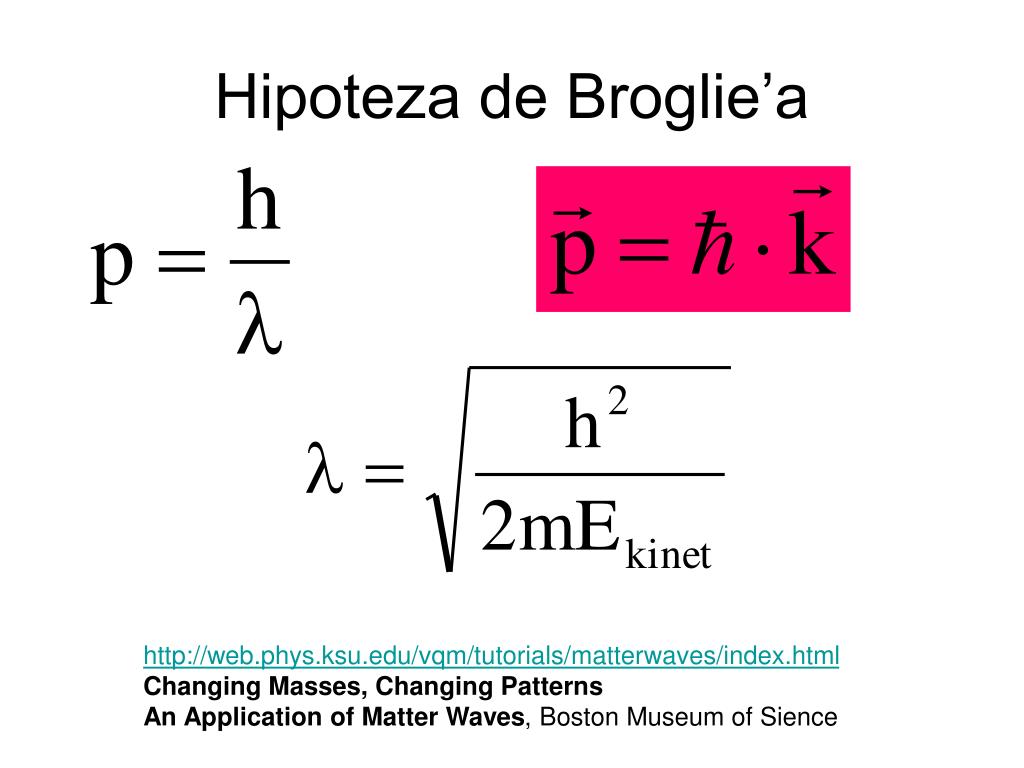
Hipoteza de Broglie’a. Interpretacja funkcji falowej Ψ. Zasada
The De Broglie hypothesis proposes that all matter exhibits wave-like properties and relates the observed wavelength of matter to its momentum. After Albert Einstein's photon theory became accepted, the question became whether this was true only for light or whether material objects also exhibited wave-like behavior.

Difference Between De Broglie Wavelength And Wavelength Relationship
În fizică, ipoteza De Broglie este afirmația că materia (orice obiect) are o natură ondulatorie ( dualitatea undă-corpuscul ). Relațiile De Broglie arată că lungimea de undă este invers proporțională cu impulsul unei particule și că frecvența este direct proporțională cu energia cinetică a particulei.

velocity of deBroglie wave YouTube
Hipoteza de Broglie'a Liceum ogólnokształcące i technikum Fizyka długość fali cząstki dualizm korpuskularno-falowy fale materii hipoteza de Broglie'a Udostępnij Wprowadzenie Przeczytaj Film samouczek Sprawdź się Dla nauczyciela Tekst: Krystyna Wosińska Politechnika Warszawska - Wydział Fizyki

Mechanika falowa podstawy Hipoteza de Broglie a Zarówno promieniowanie
Hipoteza de Broglie'a Czy to nie ciekawe? Stwór na tym zdjęciu wygląda, jak groźny przybysz z kosmosu. Ale widujemy te stworzenia często, jak całymi stadami latają nad miską z owocami. To maleńka muszka owocowa, sfotografowana za pomocą mikroskopu elektronowego. W mikroskopie tym zamiast światłem, badany obiekt „oświetla" się elektronami.
PPT Mechanika Kwantowa PowerPoint Presentation, free download ID
Well, let's first review how we define the phase velocity. The phase velocity is the speed at which a particular point on the wave moves through space. This particular point has a constant phase, so let's set the phase in the standard definition of a wave, Ψ(x, t) = A sin(kx − ωt) (5.6.3) (5.6.3) Ψ ( x, t) = A sin ( k x − ω t) equal.
II.4. Ipoteza de Broglie. Difracția electronilor. Fizichim
Prince Louis-Victor Pierre Raymond de Broglie. The Nobel Prize in Physics 1929. Born: 15 August 1892, Dieppe, France. Died: 19 March 1987, Paris, France. Affiliation at the time of the award: Sorbonne University, Institut Henri Poincaré, Paris, France. Prize motivation: "for his discovery of the wave nature of electrons".

Hipótesis de De Broglie Examen Fisica Selectividad YouTube
While this equation was specifically for waves, de Broglie, using his hypothesis that particles can act like waves, combined the equations: E = m c 2 = h ν. Where E is energy, m is mass, c is the.

Louis de Broglie Alchetron, The Free Social Encyclopedia
W 1924 roku Louis de Broglie (1892-1987) zaproponował nową hipotezę, zakładającą, że elektrony i inne cząstki materii mogą zachowywać się jak fale. Ideę tę nazywamy hipotezą de Broglie'a o falach materii (ang. de Broglie's hypothesis of matter waves). Hipoteza ta doprowadziła do rozwoju mechaniki kwantowej (ang.
PPT Czym jest i czym nie jest fala? PowerPoint Presentation, free
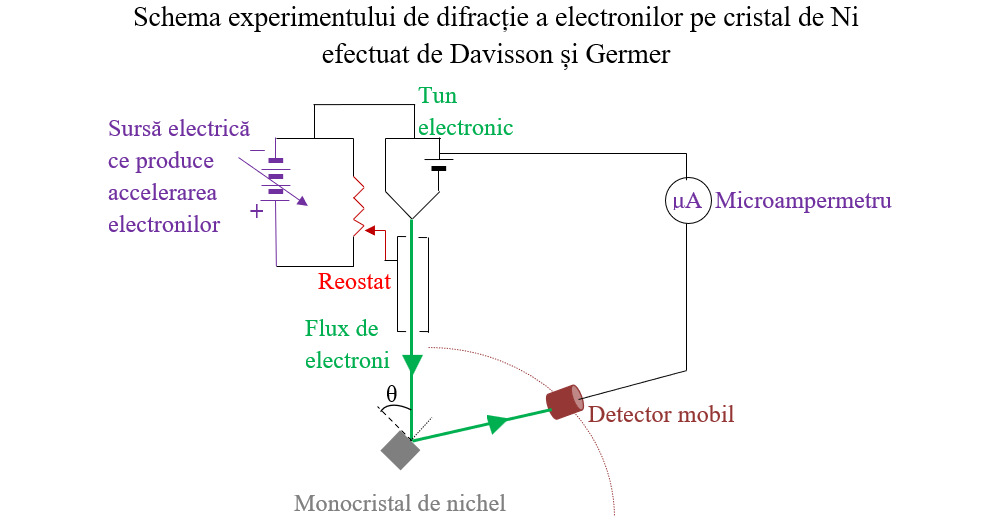
De Broglie je 1929. dobio Nobelovu nagradu za svoju teoriju (prvi put je ikada dodijeljena za doktorsku tezu), a Davisson/Germer su je zajedno osvojili 1937. za eksperimentalno otkriće difrakcije elektrona (a time i dokazivanje de Broglieove hipoteza).. Iako de Broglieova hipoteza predviđa valne dužine za bilo koju veličinu, postoje.
What are the important properties of the De Broglie's waves? Quora
Hipoteza e de Broglie tregoi se dualiteti i grimcave të valëve nuk ishte thjesht një sjellje e gabuar e dritës, por përkundrazi ishte një parim themelor i ekspozuar nga rrezatimi dhe materia.

Elementy fizyki kwantowej
Hipoteza de Broglie tregoi se dualiteti i valëve-grimcë nuk ishte thjesht një sjellje e gabuar e dritës, por më tepër ishte një parim themelor i ekspozuar nga rrezatimi dhe materia. Si i tillë, bëhet e mundur që të përdoren ekuacionet e valës për të përshkruar sjelljen materiale, për aq kohë sa të zbatohet siç duhet gjatësia e valës de Broglie.

De Broglie's wavelength YouTube
Fizicianul francez Louis de Broglie (1892-1987) a formulat, în 1923, ipoteza că orice particulă în mișcare are și proprietăți ondulatorii. 🔦 Observație Louis de Broglie a primit Premiul Nobel în 1929. Legătura dintre frecvența undei (ν) de energia particulei (E) este dată de relația:

Contoh Soal Fisika Kuantum Kelas 12
Louis de Broglie, (born August 15, 1892, Dieppe, France—died March 19, 1987, Louveciennes), French physicist best known for his research on quantum theory and for predicting the wave nature of electrons.He was awarded the 1929 Nobel Prize for Physics.. Early life. De Broglie was the second son of a member of the French nobility. From the Broglie family, whose name is taken from a small town.

De Broglie Hipotezi1 [Temel Kavramlar] YouTube
De Broglie successfully provided the explanation to Bohr's assumption by his hypothesis. Today we know that every particle exhibits both matter and wave nature. This is called wave-particle duality. The concept that matter behaves like wave is called the de Broglie hypothesis, named after Louis de Broglie, who proposed it in 1924.